6. SUBSTANCE PROFILES FOR THE PERSISTENT ORGANIC POLLUTANTS
* 6.1 ALDRIN
* 6.2 CHLORDANE
* 6.3 DDT
* 6.4 DIELDRIN
* 6.5 POLYCHLORINATED DIBENZO-p- DIOXINS AND FURANS
* 6.6 ENDRIN
* 6.7 HEXACHLOROBENZENE
* 6.8 HEPTACHLOR
* 6.9 MIREX
* 6.10 POLYCHLORINATED BIPHENYLS
* 6.11 TOXAPHENE
Information on countries that have taken action to ban or severely restrict compounds
is derived from multiple sources dating back to 1987. This information needs to be
verified and updated.
6.1 ALDRIN Chemical properties:
CAS chemical name: 1,2,3,4,10,10-Hexachloro-1,4,4a,5,8,8a-hexahydro-1,4:5,8-dimethanonaphthalene.
Synonyms and Trade Names (partial list): Aldrec, Aldrex, Aldrex 30, Aldrite, Aldrosol,
Altox, Compound 118, Drinox, Octalene, Seedrin.
CAS No.: 309-00-2; molecular formula: C12H8Cl6; formula weight: 364.92
Appearance: White, odourless crystals when pure; technical grades are tan to dark brown
with a mild chemical odour.
Properties: Melting point: 104 C(pure), 49-60 C(technical); boiling point: 145 C at 2 mm
Hg; KH: 4.96 x 10-4 atm m3/mol at 25 C; log KOC: 2.61, 4.69; log KOW: 5.17-7.4; solubility
in water: 17-180 µg/L at 25 C; vapour pressure: 2.31 x 10-5 mm Hg at 20 C.
Aldrin is a pesticide used to control soil insects such as termites, corn rootworm,
wireworms, rice water weevil, and grasshoppers. It has been widely used to protect crops
such as corn and potatoes, and has been effective to protect wooden structures from
termites. Aldrin is readily metabolized to dieldrin by both plants and animals. As a
result, aldrin residues are rarely found in foods and animals, and then only in small
amounts. It binds strongly to soil particles and is very resistant to leaching into
groundwater. Volatilization is an important mechanism of loss from the soil. Due to
its persistent nature and hydrophobicity, aldrin is known to bioconcentrate, mainly
as its conversion products. Aldrin is banned in many countries, including Bulgaria,
Ecuador, Finland, Hungary, Israel, Singapore, Switzerland and Turkey. Its use is severely
restricted in many countries, including Argentina, Austria, Canada, Chile, the EU, Japan,
New Zealand, the Philippines, USA, and Venezuela. Aldrin is toxic to humans; the lethal
dose of aldrin for an adult man has been estimated to be about 5g, equivalent to 83 mg/kg
body weight. Signs and symptoms of aldrin intoxication may include headache, dizziness,
nausea, general malaise, and vomiting, followed by muscle twitchings, myoclonic jerks,
and convulsions. Occupational exposure to aldrin, in conjunction with dieldrin and endrin,
was associated with a significant increase in liver and biliary cancer, although the
study did have some limitations, including a lack of quantitative exposure information.
There is limited information that cyclodienes, such as aldrin, may affect immune
responses. The acute oral LD50 for aldrin in laboratory animals is in the range of
33 mg/kg body weight for guinea pigs to 320 mg/kg body weight for hamsters. Reproductive
effects in rats were observed when pregnant females were dosed with 1.0 mg/kg aldrin
subcutaneously. Offspring experienced a decrease in the median effective time for incisor
teeth eruption and increase in the median effective time for testes descent. There is, as
yet, no evidence of a teratogenic potential for aldrin. IARC has concluded that there is
inadequate evidence for the carcinogenicity of aldrin in humans, and there is only limited
evidence in experimental animals. Aldrin is therefore not classifiable as to its
carcinogenicity in humans (IARC, Group 3). Aldrin has low phytotoxicity, with plants
affected only by extremely high application rates. The toxicity of aldrin to aquatic
organisms is quite variable, with aquatic insects being the most sensitive group of
invertebrates. The 96-h LC50 values range from 1-200 µg/L for insects, and from 2.2-53
µg/L for fish. Long term and bioconcentration studies are performed primarily using
dieldrin, the primary conversion product of aldrin. In a model ecosystem study, only 0.5%
of the original radioactive aldrin was stored as aldrin in the mosquitofish
(Gambusia affinis), the organism at the top of the model food chain. The acute
toxicity of aldrin to avian species varies in the range of 6.6 mg/kg for bobwhite
quail to 520 mg/kg for mallard ducks. Aldrin treated rice is thought to have been the
cause of deaths of waterfowl, shorebirds and passerines along the Texas Gulf Coast,
both by direct poisoning by ingestion of aldrin treated rice and indirectly by consuming
organisms contaminated with aldrin. Residues of aldrin were detected in all samples of
bird casualties, eggs, scavengers, predators, fish, frogs, invertebrates and soil.
As aldrin is readily and rapidly converted to dieldrin in the environment its, fate is
closely linked to that of dieldrin. Aldrin is readily metabolised to dieldrin in both
animals and plants, and therefore aldrin residues are rarely present in animals and
then only in very small amounts. Residues of aldrin have been detected in fish in Egypt,
the average concentration was 8.8 µg/kg, and a maximum concentration of 54.27 µg/kg.
The average daily intake of aldrin and dieldrin was calculated to be 19µg/person in
India, and 0.55 µg/person in Vietnam. Dairy products, such as milk and butter, and
animal meats are the primary sources of exposure.
6.2 CHLORDANE
Chemical properties:
CAS Chemical Name: 1,2,4,5,6,7,8,8-octachloro-2,3,3a,4,7,7a-hexahydro-4,7-methano-1H-
indene
Trade names: (partial list): Aspon, Belt, Chloriandin, Chlorkil, Chlordane, Corodan,
Cortilan-neu, Dowchlor, HCS 3260, Kypchlor, M140, Niran, Octachlor, Octaterr, Ortho-Klor,
Synklor, Tat chlor 4, Topichlor, Toxichlor, Veliscol-1068.
CAS No.: 57-74-9; molecular formula: C10H6Cl8; formula weight: 409.78
Appearance: colourless to yellowish-brown viscous liquid with an aromatic, pungent odour
similar to chlorine;
Properties: Melting point: <25 C; boiling point: 165 C at 2 mm Hg;
KH: 4.8 x 10-5 atm m3/mol at 25 C; log KOC: 4.58-5.57; log KOW: 6.00; solubility in water:
56 ppb at 25 C; vapour pressure: 10-6 mm Hg at 20 C. Chlordane is a broad spectrum
contact insecticide that has been used on agricultural crops including vegetables, small
grains, maize, other oilseeds, potatoes, sugarcane, sugar beets, fruits, nuts, cotton
and jute. It has also been used extensively in the control of termites. Chlordane is
highly insoluble in water, and is soluble in organic solvents. It is semi-volatile and
can be expected to partition into the atmosphere as a result. It binds readily to aquatic
sediments and bioconcentrates in the fat of organisms as a result of its high partition
coefficient (log KOW = 6.00). Action to ban the use of chlordane has been taken in
Austria, Belgium, Bolivia, Brazil, Chile, Columbia, Costa, Rica, Denmark, Dominican
Republic, EU, Kenya, Korea, Lebanon, Liechtenstein, Mozambique, Netherlands, Norway,
Panama, Paraguay, Philippines, Poland, Portugal, Santa Lucia, Singapore, Spain, Sweden,
Switzerland, Tonga, Turkey, United Kingdom, Yemen and Yugoslavia. Its use is severely
restricted or limited to non-agricultural uses in Argentina, Belize, Bulgaria, Canada,
China, Cyprus, Dominica, Egypt, Honduras, Indonesia, Israel, Mexico, New Zealand,
South Africa, Sri Lanka, USA and Venezuela. Early studies on occupational exposure
found no toxic effects in workers involved in the production of chlordane with up to
15 years of exposure. In a survey of 1105 workers associated with pest control, most
of whom used chlordane, however, only three attributed illness to it (mild dizziness,
headache, weakness). Chlordane exposure has not been associated with increased risk
of mortality from cancer. Significant changes in the immune system were reported in
individuals who complained of health effects which they associated with chlordane
exposure. Acute oral toxicity for chlordane in laboratory animals ranges from
83 mg/kg for pure cis-chlordane in rats to 1720 mg/kg for hamsters. Subchronic (
90 day) inhalation exposure in rats and monkeys at doses up to 10 mg/m3 resulted in
increases in the concentration of cytochrome P-450 and microsomal protein in rats.
The results of this study provide a no-effect level in the rat of approximately 0.1
mg/m3 and in excess of in 10 mg/m3 the monkey. Mice were fed diets containing chlordane
for 6 generations. At 100 mg/kg, viability was decreased in the first and second
generation, and no offspring were produced in the third generation. At 50 mg/kg,
viability was decreased in the third and fourth generation, and at 25 mg/kg no
statistically significant effects were observed after 6 generations. Offspring of
rabbits administered chlordane orally on the 5th - 18th days of gestation did not
exhibit changes in behaviour, appearance or body weight were observed, and no
teratogenic effects were reported. IARC has concluded that, while there is inadequate
evidence for the carcinogenicity of chlordane in humans, there is sufficient evidence
in experimental animals. IARC has classified chlordane as a possible human carcinogen (
Group 2B). The acute toxicity of chlordane to aquatic organisms is quite variable,
with 96-hour LC50 values as low as 0.4 µg/L for pink shrimp. The acute oral LD50 to 4-5
month old mallard ducklings was 1200 mg/kg body weight. The LC50 for bobwhite quail fed
chlordane in their diet for 10 weeks was 10 mg/kg diet. The half-life of chlordane in
soil has been reported to be approximately one year. This persistence, combined with a
high partition coefficient, provides the necessary conditions for chlordane to
bioconcentrate in organisms. Bioconcentration factors of 37,800 for fathead minnows
and 16,000 for sheepshead minnow have been reported. Data suggest that chlordane is
bioconcentrated (taken up directly from the water) as opposed to being bioaccumulated
(taken up by water and in food). The chemical properties of chlordane (low water
solubility, high stability, and semi-volatility) favour its long range transport, and
chlordane has been detected in arctic air, water and organisms. Chlordane exposure
may occur through food but, due to its highly restricted uses, this route does not
appear to be a major pathway of exposure. The isomer gamma-chlordane was detected in
only 2 (8.00 and 36.17 µg/kg wet weight) of 92 samples of Egyptian fish and in 2 of 9
samples (2.70 and 0.48 ppb) of food products imported into Hawaii from western Pacific
rim countries. Chlordane has been detected in indoor air of residences of both Japan
and the US. Exposure to chlordane in the air may be an important source of exposure to
the US population. Mean levels detected in the living areas of 12 homes in New Jersey
prior to and after treatment for termites ranged from 0.14 to 0.22 µg/m3, respectively.
Mean levels in non-living areas (crawl spaces and unfinished basements) were higher;
0.97 µg/m3 before treatment and 0.91 µg/m3 after treatment. Levels detected in New
Jersey homes before and after regulations restricting chlordane use fell from 2.6 to
0.9 µg/m3.
6.3 DDT
Chemical properties:
CAS Chemical Name: 1,1'-(2,2,2-Trichloroethylidene)bis(4-chlorobenzene)
Synonyms and Trade Names (partial list): Agritan, Anofex, Arkotine, Azotox, Bosan Supra,
Bovidermol, Chlorophenothan, Chloropenothane, Clorophenotoxum, Citox, Clofenotane, Dedelo,
Deoval, Detox, Detoxan, Dibovan, Dicophane, Didigam, Didimac, Dodat, Dykol, Estonate,
Genitox, Gesafid, Gesapon, Gesarex, Gesarol, Guesapon, Gyron, Havero-extra, Ivotan,
Ixodex, Kopsol, Mutoxin, Neocid, Parachlorocidum, Pentachlorin, Pentech, PPzeidan,
Rudseam, Santobane, Zeidane, Zerdane.
CAS No.: 50-29-3; molecular formula: C14H9Cl5; formula weight: 354.49.
Appearance: Odourless to slightly fragrant colourless crystals or white powder.
Properties: Melting point: 108.5 C; boiling point: 185 C at 0.05 mm Hg (decomposes);
KH: 1.29 x 10-5 atmám3/mol at 23 C; log KOC: 5.146-6.26; log KOW: 4.89-6.914; solubility
in water: 1.2-5.5 µg/L at 25 C. DDT was widely used during the Second World War to
protect the troops and civilians from the spread of malaria, typhus and other vector
borne diseases. After the war, DDT was widely used on a variety of agricultural crops
and for the control of disease vectors as well. It is still being produced and used for
vector control. Growing concern about adverse environmental effects, especially on wild
birds, led to severe restrictions and bans in many developed countries in the early 1970s.
The largest agricultural use of DDT has been on cotton, which accounted for more than 80%
of the US use before its ban there in 1972. DDT is still used to control mosquito vectors
of malaria in numerous countries. DDT is highly insoluble in water and is soluble in most
organic solvents. It is semi-volatile and can be expected to partition into the atmosphere
as a result. Its presence is ubiquitous in the environment and residues have even been
detected in the arctic. It is lipophilic and partitions readily into the fat of all living
organisms and has been demonstrated to bioconcentrate and biomagnify. The breakdown
products of DDT, 1,1-dichloro-2,2-bis(4-chlorophenyl)ethane (DDD or TDE) and 1,1-dichloro-2,
2bis(4-chlorophenyl)ethylene) (DDE), are also present virtually everywhere in the
environment and are more persistent than the parent compound. The use of DDT has been
banned in 34 countries and severely restricted in 34 other countries. The countries that
have banned DDT include Argentina, Australia, Bulgaria, Canada, Colombia, Cyprus,
Ethiopia, Finland, Hong Kong, Japan, Lebanon, Mozambique, Norway, Switzerland, and the
USA. Countries that have severely restricted its use include Belize, Ecuador, the EU,
India, Israel, Kenya, Mexico, Panama, and Thailand. DDT has been widely used in large
numbers of people who were sprayed directly in programs to combat typhus, and in tropical
countries to combat malaria. Dermal exposure to DDT has not been associated with illness
or irritation in a number of studies. Studies involving human volunteers who ingested DDT
for up to 21 months did not result in any observed adverse effects. A non-significant
increase in mortality from liver and biliary cancer and a significant increase in
mortality from cerebrovascular disease has been observed in workers involved in the
production of DDT. There is some evidence to suggest that DDT may be suppressive to the
immune system, possibly by depressing humoral immune responses. Perinatal administration
of weakly estrogenic pesticides such as DDT produces estrogen-like alterations of
reproductive development, and there is also limited data that suggest a possible
association between organochlorines, such as DDT and its metabolite DDE, and risk
of breast cancer. DDT is not highly acutely toxic to laboratory animals, with acute
oral LD50 values in the range of 100 mg/kg body weight for rats to 1,770 mg/kg for
rabbits. In a six generation reproduction study in mice, no effect on fertility,
gestation, viability, lactation or survival were observed at a dietary level of 25 ppm.
A level of 100 ppm produced a slight reduction in lactation and survival in some
generations, but not all, and the effect was not progressive. A level of 250 ppm produced
clear adverse reproductive effects. In both these and other studies, no evidence of
teratogenicity has been observed. IARC has concluded that while there is inadequate
evidence for the carcinogenicity of DDT in humans, there is sufficient evidence in
experimental animals. IARC has classified DDT as a possible human carcinogen (Group 2B).
DDT is highly toxic to fish, with 96-hour LC50 values in the range of 0.4 µg/L in shrimp
to 42 µg/L in rainbow trout. It also affects fish behaviour. Atlantic salmon exposed to
DDT as eggs experienced impaired balance and delayed appearance of normal behaviour
patterns. DDT also affects temperature selection in fish. DDT is acutely toxic to birds
with acute oral LD50 values in the range of 595 mg/kg body weight in quail to 1,334 mg/kg
in pheasant, however it is best known for its adverse effects on reproduction, especially
DDE, which causes egg shell thinning in birds with associated significant adverse impact
on reproductive success. There is considerable variation in the sensitivity of bird
species to this effect, with birds of prey being the most susceptible and showing
extensive egg shell thinning in the wild. American kestrels were fed day old cockerels
injected with DDE. Residues of DDE in the eggs correlated closely with the dietary DDE
concentration and there was a linear relationship between degree of egg shell thinning
and the logarithm of the DDE residue in the egg. Data collected in the field has confirmed
this trend. DDT (in conjunction with other halogenated aromatic hydrocarbons) has been
linked with feminization and altered sex-ratios of Western Gull populations off the coast
of southern California, and Herring Gull populations in the Great Lakes. DDT and related
compounds are very persistent in the environment, as much as 50% can remain in the soil
10-15 years after application. This persistence, combined with a high partition
coefficient (log KOW = 4.89-6.91) provides the necessary conditions for DDT to
bioconcentrate in organisms. Bioconcentration factors of 154,100 and 51,335 have been
recorded for fathead minnows and rainbow trout, respectively. It has been suggested that
higher accumulations of DDT at higher trophic levels in aquatic systems results from a
tendency for organisms to accumulate more DDT directly from the water, rather than by
biomagnification. The chemical properties of DDT (low water solubility, high stability
and semi-volatility) favour its long range transport and DDT and its metabolites have
been detected in arctic air, water and organisms. DDT has also been detected in virtually
all organochlorine monitoring programs and is generally believed to be ubiquitous
throughout the global environment. DDT and its metabolites have been detected in food
from all over the world and this route is likely the greatest source of exposure for
the general population . DDE was the second most frequently found residue (21%) in a
recent survey of domestic animal fats and eggs in Ontario, Canada, with a maximum residue
of 0.410 mg/kg. Residues in domestic animals, however, have declined steadily over the
past 20 years. In a survey of Spanish meat and meat products, 83% of lamb samples tested
contained at least one ofthe DDT metabolites investigated, with a mean level of 25 ppb.
An average of 76.25 ppb p,p'-DDE was detected in fish samples from Egypt. DDT was the
most common organochlorine detected in foodstuffs in Vietnam with mean residue
concentrations of 3.2 and 2.0 µg/g fat in meat and fish, respectively. The estimated
daily intake of DDT and its metabolites in Vietnam was 19 µg/person/day. Average residues
detected in meat and fish in India were 1.0 and 1.1 µg/g fat respectively, with an
estimated daily intake of 48 µg/person/day for DDT and its metabolites. DDT has also
been detected in human breast milk. In a general survey of 16 separate compounds in the
breast milk of lactating mothers in four remote villages in Papua, New Guinea, DDT was
detected in 100% of samples (41), and was one of only two organochlorines detected. DDT
has also been detected in the breast milk of Egyptian women, with an average total DDT
detected of 57.59 ppb and an estimated daily intake of total DDT for breast feeding
infants of 6.90 µg/kg body weight /day. While lower than the acceptable daily intake
of 20.0 µg/kg body weight recommended by the Joing FAO/WHO Meeting on Pesticide Residues
(JMPR), its continuing presence raises serious concerns regarding potential effects on
developing infants.
6.4 DIELDRIN
Chemical properties:
CAS Chemical Name: 3,4,5,6,9,9-Hexachloro-1a,2,2a,3,6,6a,7,7a-octahydro-2,7:3,6-
dimetanonapth[2,3-b]oxirene.
Synonyms and Trade Names (partial list):Alvit, Dieldrite, Dieldrix, Illoxol, Panoram D-31,
Quintox.
CAS No.:60-57-1; molecular formula: C12H8Cl6O; formula weight: 380.91.
Appearance: A stereo-isomer of endrin, dieldrin may be present as white crystals or pale
tan flakes, odourless to mild chemical odour. Properties: Melting point: 175-176 C;
boiling point: decomposes; KH: 5.8 x 10-5 atmám3/mol at 25 C; log KOC: 4.08-4.55; log
KOW: 3.692-6.2; solubility in water: 140 µg/L at 20 C; vapour pressure: 1.78 x 10-7 mm
Hg at 20 C. Dieldrin has been used in agriculture for the control of soil insects and
several insect vectors of disease but this latter use has been banned in a number of
countries due to environmental and human health concerns. Principle contemporary uses
are restricted to control termites and wood borers and against textile pests (WHO, 1989).
Dieldrin binds strongly to soil particles and hence is very resistant to leaching into
groundwater. Volatilization is an important mechanism of loss from the soil and, because
of its persistent nature and hydrophobicity, dieldrin is known to bioconcentrate. Action
to ban dieldrin has been taken in many countries, including Bulgaria, Ecuador, the EU,
Hungary, Israel, Portugal, Singapore, Sweden, and Turkey. Its use is severely restricted
in numerous countries, including Argentina, Austria, Canada, Colombia, Cyprus, India,
Japan, New Zealand, Pakistan, USA and Venezuela. In a study using human volunteers, the
subjects received dieldrin daily for 2 years. All the volunteers continued in excellent
health, and clinical, physiological and laboratory findings remained essentially unchanged
through the exposure period and an 8 month follow up. In a study of workers from a plant
involved in the manufacture of aldrin, dieldrin and endrin, a statistically significant
increase in liver and biliary tract cancers was observed, although the study did have some
limitations, including lack of quantitative exposure information. In laboratory studies,
acute oral LD50 values in the range of 37 mg/kg body weight in rats to 330 mg/kg in
hamsters have been found for dieldrin. As with other organochlorine compounds, the liver
is the major target organ in rats, with effects that included increased liver/body weight
ratio, hypertrophy and histopathological changes. The no observed adverse effect level
(NOAEL) in rats is 0.5 mg/kg diet, equal to 0.025 mg/kg body weight/day. When rats were
fed dieldrin in their diet over three generations, no changes in reproductive capacity
were observed at any dose level tested. A NOAEL of 2 mg dieldrin /kg diet has been set
for reproduction in rats. There was no evidence for teratogenic potential in studies in
rats, mice or rabbits using oral doses of up to 6 mg/kg body weight. Abnormal development
and fetotoxicity were observed in hamsters and mice, however, these results are unlikely
to be of significance in view of the maternal toxicity noted at the high dose levels.
There is limited evidence that cyclodienes such as dieldrin may affect immune responses.
IARC has concluded that there is inadequate evidence for the carcinogenicity of dieldrin
in humans, and limited evidence in experimental animals and has been classified by IARC
in Group 3. Dieldrin has low phytotoxicity. Plants are affected only by application rates
much higher than suggested use rates. The acute toxicity of dieldrin is quite variable for
aquatic invertebrates, with insects being the most sensitive group (values range from
0.2-40 µg/L). It is highly toxic to most species of fish tested in the laboratory (values
range from 1.1-41 µg/L). Acute toxicity of dieldrin in frogs (96-h LC50) ranged from 8.7
µg/L for Rana catesbeiana tadpoles to 71.3µg/L for the tadpoles of Rana pipiens. Spinal
deformities in embryo-larval tests were observed at concentrations as low as 1.3 µg/L for
Xenopus laevis after a 10 day exposure. The acute toxicity of dieldrin to avian species
varies widely, with acute oral LD50 values in the range of 26.6 in pigeons to 381 mg/kg
in mallard ducks. Mallard ducklings were exposed to dieldrin in the diet for 24 days. A
24 d NOAEL of 0.3µg dieldrin/g diet, based on growth impairment, was determined.
Reproduction success in birds has not been consistently affected in the absence of
maternal toxicity. The acute LD50 of dieldrin to four species of voles range from
100 to 210 mg/kg body weight, suggesting that these microtine rodents are less
susceptible than laboratory rodents to dieldrin. In another study, white tailed
deer (Odocoileus virginianus) were fed diet containing dieldrin for up to 3 years.
Adult survival was not affected, and fertility and in utero mortality was comparable
for all groups. Fawns from treated does were smaller at birth, experienced greater
postpartum mortality and weight gain was reduced. Blesbuck (Damaliscuc dorcas phillipsi)
were fed diets containing dieldrin for 90 days. None of the animals fed 5 or 15 mg/kg diet
died during the study period, but all animals at the higher dose levels died within 24 days.
The half life of dieldrin in temperate soils is approximately 5 years. This persistence,
combined with high lipid solubility, provides the necessary conditions for dieldrin to
bioconcentrate and biomagnify in organisms. Bioconcentration factors of 12,500 and 13,300
have been reported for guppies and sculpins, respectively. It is likely that dieldrin is
bioconcentrated by aquatic organisms rather than bioaccumulated. Dieldrin's chemical
properties (low water solubility, high stability, and semi-volatility) favour its long
range transport, and dieldrin has been detected in arctic air, water and organisms.
Dieldrin residues have been detected in air, water, soil, fish, birds and mammals,
including humans and human breast milk. As aldrin is readily and rapidly converted
to dieldrin in the environment and in organisms, the levels of dieldrin detected likely
reflect the total concentrations of both compounds. In Egypt, the estimated dietary intake
of dieldrin by breast fed infants of 1.22 µg/kg body weight/ day. Diet is the main source
of exposure to the general public. Dieldrin was the second most common pesticide detected
in a survey of US pasteurized milk, detected in 172 of the 806 composite samples tested,
with a maximum level of 0.003 ppm. Dieldrin residues were detected in 9 of 602 (1.5%)
samples of domestic animal fats and eggs in Canada, with a maximum of 0.050 mg/kg.
Dieldrin was also detected in Spanish meat, residues of 20 to 40 ppb were detected in the
fat of 8 to 15% of pork products (meat, cured sausage, pork bologna) and in 28% fresh
poultry sausage. Dieldrin residues were detected in Oriental party beans at 3.45 ppb.
The average daily intake of aldrin and dieldrin in India was calculated to be 19 µg/person,
exceeding the acceptable daily intake of 6.0 µg/60 kg of body weight recommended by the
Joint FAO/WHO Meeting on Pesticide Residues (JMPR). Dairy products, such as milk and
butter, and animal meats were the primary sources of exposure. Exposure through food
intake has been estimated at 0.55 µg/person in Vietnam.
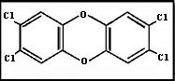
6.5 POLYCHLORINATED DIBENZO - p - DIOXINS AND FURANS
Chemical properties:
Dioxins Congener Molecular weight Vapour Water Solubility Log KOW
Group (g/molecular) Pressure (mg/m3)
(Pa X 10-3) M1CDD 218.5
73-75 295-417 4.75-5.00 D2CDD 253.0
2.47-9.24 3.75-16.7 5.60-5.75 T3CDD 287.5
1.07 8.41 6.35 T4CDD 322.0
0.00284-0.275 0.0193-0.55 6.60-7.10 P5CDD 356.4
0.00423 0.118 7.40 H6CDD 391.0
0.00145 0.00442 7.80 H7CDD 425.2
0.000177 0.0024 8.00 O8CDD 460.0
0.000953 0.000074 8.2
Polychlorinated dibenzo-para-dioxins (dioxins) and polychlorinated dibenzofurans
(furans) are two groups of planar tricyclic compounds that have very similar chemical
structures and properties. They may contain between 1 and 8 chlorine atoms; dioxins have
75 possible positional isomers and furans have 135 positional isomers. They are generally
very insoluble in water, are lipophilic and are very persistent. The chemical properties
of each of the isomers has not been elucidated, further complicating a discussion of their
properties which vary with the number of chlorine atoms present. Neither dioxins nor
furans are produced commercially, and they have no known use. They are by-products
resulting from the production of other chemicals. Dioxins may be released into the
environment through the production of pesticides and other chlorinated substances.
Furans are a major contaminant of PCBs. Both dioxins and furans are related to a
variety of incineration reactions, and the synthesis and use of a variety of chemical
products. Dioxins and furans have been detected in emissions from the incineration of
hospital waste, municipal waste, hazardous waste, car emissions, and the incineration
of coal, peat and wood. Of the 210 dioxins and furans, 17 contribute most significantly
to the toxicity of complex of mixtures. In order to facilitate a comparison mixtures,
International Toxicity Equivalency Factors (TEFs) have been assigned to individual
dioxins and furans basedon a comparison of toxicity to 2,3,7,8-tetrachlorodibenzodioxin
(2,3,7,8-TCDD). For example, 2,3,7,8-TCDF has been shown to be approximately one-tenth
as toxic as 2,3,7,8-TCDD in animal tests, and its toxic equivalent value is 0.1. TEFs are
regarded as risk management tools and they do not necessarily represent actual toxicity
with respect to all endpoints. Rather, they tend to overestimate the toxicity of
mixtures. At the present time, the only persistent effect associated with dioxin exposure
in humans is chloracne. Other health effects that have been reported include peripheral
neuropathies, fatigue, depression, personality changes, hepatitis, enlarged liver,
abnormal enzyme levels and porphyria cutanea tarda though no causal relationships were
established in every case. Results of a study on 1,520 workers known to have been exposed
to 2,3,7,8-TCDD for a period of at least one year, and with a latency of at least twenty
years between exposure and diagnosis of disease, revealed a slightly, but significantly
elevated mortality from soft tissue sarcoma and cancers of the respiratory system. As w
ith other studies, interpretation of results was limited by the small number of deaths
and by possible confounders including smoking and other occupational exposures. Two
recent studies followed a young population from the area of the Seveso, Italy industrial
accident. The first, a cancer study, examined a cohort of people aged 0-19 years living
in the accident area at the time of the accident, for the period 1977-1986. While a
consistent tendency toward increased risk was apparent, none of the relative risks were
significantly elevated. Non-significant increases in thyroid cancer and myeloid leukemia
were also observed. The study is limited, however, by the relatively short latency periods,
the definition of exposure based on place of residence and the limited number of events. The
second study examined the mortality of the same cohort of people for the same time period.
Among the exposed, mortality owing to all causes did not deviate from expectations, however,
as noted above, this study provides only limited evidence. Direct exposure of humans to
furans has been reported in two incidents of rice oil contamination by PCBs contaminated
with PCDFs, in Japan (Yusho) and Taiwan (Yucheng). While it is possible that the effects
observed in these incidents may be due to the presence of furans, the similarity of
structure, effects and mode of action of PCBs and PCDFs precludes a definite conclusion
on the causative agent. The acute oral toxicity in laboratory animals is highly variable,
with LD50 values ranging from 0.6 µg/kg body weight in guinea pigs to 1,157 µg/kg in
hamsters. Effects of dioxin exposure that are common to most, and sometimes all, species
include wasting, lymphoid involution, hepatotoxicity, chloracne and epidermal changes, and
gastric lesions. Other characteristic reponses include edema, ascites and hypopericardium
in chickens; fetal death and resorption in rats and fetal wastage, embryotoxicity and
malformations in mice. A three-generation study was conducted in which rats were fed diets
containing 2,3,7,8-TCDD. Significant decreases in fertility and neonatal survival were
observed in the f0 group receiving 0.1 µg TCDD/kg/day. At 0.01 µg TCDD/kg/day, fertility
was significantly reduced in the f1 and f2 generations. Decreases in litter size,
gestation survival and neonatal survival and growth were also observed at this dose
level. No effect on fertility, litter size at birth or post natal body weight was
observed in any generation of the 0.001 µg TCDD/kg/day group. Some teratogenic effects
have been observed in mice in association with dioxin and furan exposure, including
hydronephrosis and cleft palate The most teratogenic isomer was 2,3,4,7,8-pentachloro
dibenzofuran, with an ED50 of 36 µg/kg for cleft palate and 7 µg/kg for hydronephrosis.
Teratogenic responses observed are similar to those seen with TCDD, but theses compounds
are only 1/10 to 1/100 as potent. Dioxins, specifically 2,3,7,8-TCDD, are associated with
a variety of adverse effects on the reproductive systems of both male and female rats.
Male reproductive toxicity has included altered regulation of luteinizing hormone
secretion, reduced testicular steroidogenesis, reduced plasma androgen concentrations,
reduced testis and accessory sex organ weights, abnormal testis morphology, decreased
spermatogenesis, and reduced fertility. Signs of female reproductive toxicity included
hormonal irregularities in the oestrous cycle, reduced litter size and reduced fertility.
A review of recent literature concerning 2,3,7,8-TCDD effects on immunocompetence suggests
hat 2,3,7,8-TCDD either indirectly (in the case of T-cells) or directly (in the case of
B-cells) affects the maturational or differentiative processes of immunocompetent cells.
Studies in exposed human populations and in non-human primates have shown that halogenated
aromatic hydrocarbons produce measurable alterations in both innate and acquired immunity,
although significant deficits in immunocompetence have not been conclusively associated
with these changes. IARC has concluded that while there is inadequate evidence for the
carcinogenicity of 2,3,7,8-TCDD in humans, there is sufficient evidence in experimental
animals. IARC has classified 2,3,7,8-TCDD as a possible human carcinogen (Group 2B).
Other chlorinated dibenzodioxins (other than 2,3,7,8-TCDD) are deemed not classifiable
as to their carcinogenicity in humans. Exposure of fish to dioxins and furans results
in a delayed mortality that can continue many days post-exposure. Rainbow trout exposed
to 2,3,7,8-TCDD and to 2,3,7,8-TCDF for 28 days, followed by a 28 day depuration period
had a 56-day LC50 of 46 pg/L for TCDD, and a NOEC for TCDD based on growth and mortality
below the lowest exposure concentration of 38 pg/L. The 56-day NOEC for TCDF was
calculated to be 1.79 ng/L for mortality and 0.41 ng/L for growth. Mortality and
behavioural changes such as lethargic swimming, feeding inhibition and lack of
response to external stimuli continued after the 28 day exposure period ended.
Early life stages of fish are very sensitive to the effects of dioxins, furans,
and PCBs. Parts per trillion concentrations of these structurally related chemicals
in lake trout and rainbow trout eggs exhibit toxicity through sac fry mortality
associated with yolk sac edema and hemorrhages. Great blue heron eggs collected
from sites of low, intermediate and high contamination had levels of 2,3,7,8-TCDD
in eggs of 10 ng/kg (wet weight), 135 ng/kg and 211 ng/kg, respectively. Although
there was no effect on mortality of chicks, effects of contamination included decreased
growth with increased TCDD level, depression of skeletal growth with increased TCDD
levels and subcutaneous edema which increased with increasing PCDD and PCDF contamination.
Also observed were shortened beaks and a scarcity of down follicles in the chicks from the
more contaminated sites. Mink administered TCDD experienced the wasting syndrome associated
with TCDD intoxication and gastric lesions at higher dosages. A 28 day oral LD50 was
calculated to be 4.2 µg TCDD/kg body weight. Dioxins and furans are considered to be
very stable and persistent, as illustrated by the half life of TCDD in soil of 10-12
years. This persistence, combined with high partition coefficients (up to 8.20 for OCDD)
provides the necessary conditions for these compunds to bioconcentrate in organisms.
Bioconcentration factors of 26 707 has been reported in rainbow trout (Salmo gairdneri)
exposed to 2,3,7,8-TCDD. The chemical properties of dioxins and furans (low water
solubility, high stability and semi-volatility) favour their long range transport
and these compounds have been detected in arctic organisms. As with most other
organochlorines, food is a major source of dioxins and furans in the general population,
with food of animal origin contributing the most to human body burdens. In a survey of
dioxins in US food, total PCDD/Fs ranged from 0.42 ppt to 61.8 ppt (wet weight) (total
TEq range: 0.02 to 1.5 ppt). The estimated daily intake for adults ranged from 0.3 to
3.0 pg TEqs/kg body weight, and for breast fed infants the range was 35.3 to 52.6 pg
TEqs/kg body weight. Recent estimates of adult average daily intake for Canada, Germany
and the Netherlands are 1.52, 2 and 1 pg TEQ/kg bodyweight, respectively. These are below
the TDI of 10 pg/kg body weight for lifetime exposure estimated by WHO.
6.6 ENDRIN
Chemical properties:
CAS Chemical Name: 3,4,5,6,9,9,-Hexachloro-1a,2,2a,3,6,6a,7,7a-octahydro-2,7:3,6-dimeth
anonaphth[2,3-b]oxirene.
Synonyms and Trade Names (partial list): Compound 269, Endrex, Hexadrin, Isodrin Epoxide,
Mendrin, Nendrin.
CAS No.: 72-20-8; molecular formula: C12H8Cl6O; formula weight: 380.92.
Appearance: White, odourless, crystalline solid when pure; light tan colour with faint
chemical odour for technical grade.
Properties: Melting point: 200 C; boiling point: 245 C (decomposes); KH: 5.0 x 10-7
atmám3/molecular; log KOW: 3.209-5.339; solubility on water: 220-260 µg/L at 25 C; vapour
pressure: 7 x 10-7 mm Hg at 25 C. Endrin is a foliar insecticide used mainly on field
crops such as cotton and grains. It has also been used as a rodenticide to control mice
and voles. It is rapidly metabolised by animals and does not accumulate in fat to the same
extent as other compounds with similar structures. It can enter the atmosphere by
volatilization, and can contaminate surface water from soil run-off. Endrin is banned
in many countries, including Belgium, Cyprus, Ecuador, Finland, Israel, Philippines,
Singapore, Thailand and Togo. Its use is severely restricted in many countries, including
Argentina, Canada, Chile, Colombia, the EU, India, Japan, New Zealand, Pakistan, USA,
and Venezuela. A study of workers involved in the production of aldrin, dieldrin and
endrin did not find endrin in the blood of workers, except in cases of accidental,
acute over-exposure. These findings are in agreement with results of a study of 71
workers in an endrin plant in the USA. Data on absenteeism, results of liver function
tests, blood chemistry, blood morphology, urine analysis, occurrence of sensitization,
the incidence and pattern of diseases including the occurrence of malignant growth
showed no difference between workers exposed to endrin and other chemical plant operators.
A study of workers involved in the manufacture of aldrin, dieldrin and endrin found a
statistically significant increase in liver and biliary tract cancers, although the
study did have some limitations such as lack of quantitative exposure information.
There is limited evidence that cyclodienes such as endrin may also depress immune
responses. The acute oral LD50 of endrin is in the range of 3 mg/kg body weight in
monkeys to 36 mg/kg in guinea pigs. Male and female Long-Evans rats were fed endrin
in the diet over three generations. No difference in appearance, behaviour, body weight,
or number or size of litters was observed. The weights of liver, kidneys and brain were
normal, and no histopathological abnormalities were observed in third generation
weanlings. Significant increased mortality of pups in the second and third generations
of rats fed 3 mg/kg was noted. Endrin was not teratogenic at levels that did not cause
maternal toxicity. Endrin is metabolised rapidly by animals, and very little is
accumulated in fat compared to compounds of similar structure (including its stereoisomer
dieldrin). The formation of anti-12-hydroxyendrin is considered to be the major route
of metabolism of endrin. IARC has concluded that there is inadequate evidence for the
carcinogenicity of endrin in humans, and there is only limited evidence in experimental
animals. Endrin is therefore not classifiable as to its carcinogenicity in humans
(Group 3). Endrin is highly toxic to fish, with most LC50 values below 1.0 µg/L.
Sheepshead minnows embryos exposed for 23 weeks to 0.31 and 0.72 µg/L hatched early,
and all those exposed to 0.72 µg/L died by the ninth day of their exposure, while those
exposed at 0.31 µg/L were initially stunted and some died. The reproductive ability of the
survivors of the 0.31 µg/L was impaired. No significant effects were observed at an
exposure concentration of 0.12 µg/L. The lowest observed adverse effect level (LOAEL)
for aquatic organisms was 30 ng/L over 20 days for reproduction in mysid shrimp.
Reproduction in male and female mallard ducks was not impaired by diets containing
0, 0.5 or 3.0 mg/kg. The half life of endrin in soil may be up to 12 years, depending
on local conditions. This persistence, combined with a high partition coefficient
(log KOW = 3.21-5.340), provides the necessary conditions for endrin to bioconcentrate
in organisms. A bioconcentration factor of 6,400 was recorded for sheepshead minnows
exposed to endrin from embryonic stage through adulthood. Bluegill sunfish exposed to
water containing 14C-labelled endrin took up 91% of the radio-labelled endrin with in
48 hours, with a half life of loss from the tissues of approximately four weeks.
Leiostomus xantharus exposed to 0.05 µg/L for 5 months had a tissue residue level of
78 µg/kg tissue. After 18 days in uncontaminated water, no residues were detected,
suggesting that endrin disappears rapidly from this organism. The chemical properties
of endrin (low water solubility, high stability in the environment, and semi-volatility)
favour its long range transport, and it has been detected in arctic freshwater. The main
source of endrin exposure to the general population is residues in food however,
contemporary intake is generally below the acceptable daily intake of 0.0002 mg/kg
body weight recommended by the Joint FAO/WHO Meeting on Pesticide Residues (JMPR).
Recent food surveys have generally not included endrin, and hence recent monitoring
data are not available.
6.7 HEXACHLOROBENZENE
Chemical properties:
CAS Chemical Name: hexachlorobenzene
Trade names: (partial list): Amaticin, Anticarie, Bunt-cure, Bunt-no-more, Co-op hexa,
Granox, No bunt, Sanocide, Smut-go, Sniecotox
CAS No.: 118-74-1; molecular formula: C6Cl6; formula weight: 284.78;
Appearance: White monoclinic crystals or crystalline solid
Properties: Melting point: 227-230 C; boiling point: 323-326 C (sublimes); KH: 7.1 x
10-3 atm m3/mol at 20 C; log KOC: 2.56-4.54; log KOW: 3.03-6.42; Solubility in water:
40 µg/L at 20 C; vapour pressure: 1.089 x 10-5 mm Hg at 20 C. Hexachlorobenzene (HCB)
is a fungicide that was first introduced in 1945 for seed treatment, especially for
control of bunt of wheat. HCB is also a byproduct of the manufacture of industrial
chemicals including carbon tetrachloride, perchlorethylene, trichloroethylene and
pentachlorbenzene. It is a known impurity in several pesticide formulations, including
pentachlorophenol and dicloram and may be present as an impurity in others. HCB is
highly insoluble in water, and is soluble in organic solvents. It is quite volatile and
can be expected to partition into the atmosphere as a result. It is very resistant to
breakdown and has a high partition coefficient (KOW=3.03-6.42), and is known to
bioconcentrate in the fat of living organisms as a result. HCB is banned in Austria,
Belgium, Czechoslovakia, Denmark, the EU, Germany, Hungary, Liechtenstein, Netherlands,
Panama, Switzerland, Turkey, United Kingdom and the USSR. It is severely restricted or
has been voluntarily withdrawn in Argentina, New Zealand, Norway and Sweden. The most
notable episode involving the effects of HCB on humans involves the ingestion of HCB t
reated seed grain in eastern Turkey between 1954 and 1959. The patients who ingested the
treated seed experienced a range of symptoms including photosensitive skin lesions,
hyperpigmentation, hirsutism, colic, severe weakness, porphyrinuria, and debilitation.
Approximately 3,000-4,000 people developed porphyria turcica, a disorder of haem bio
synthesis. Mortality was up to 14%. Mothers who ingested the seeds passed the HCB to t
heir children by placental transfer and through maternal milk. Children born to these
women developed "pembe yara" or pink sore, with a reported mortality rate of approxima
tely 95%. A study of 32 individuals twenty years after the outbreak showed that porphy
ria can persist years after the ingestion of HCB. A small cross-sectional study of wor
kers exposed to HCB did not find any evidence of cutaneous porphyria or any other adve
rse effects associated with exposure of 1 to 4 years. The acute toxicity of HCB to la
boratory animals is quite low, with acute oral LD50 values in the range of more than 2
,600 mg/kg body weight in rabbits and 4,000 mg/kg in mice. Porphyria, skin lesions, hy
perexcitability and changes in weight, enzyme activities and morphology of the liver h
ave been reported in association with subchronic toxicity of HCB. HCB has also been re
ported to stimulate the immune system in rats, and suppress the immune system of mice.
HCB has also been reported to produce adverse effects on reproduction and reproductiv
e tissue. Female rats fed HCB in the diet experienced offspring mortality, with a 21 d
ay LD50 of 100 ppm. A four-generation reproduction study in rats fed HCB in the diet w
as conducted. HCB affected reproduction by reducing the number of litters whelped, lit
ter size and the number of pups surviving to weaning. In a separate study, HCB at a co
ncentration of 100 mg/kg body weight/day was associated with cleft palate and some kid
ney malformations in CD-1 mice. HCB exposure in several studies in cynomologous monkey
s has resulted in degenerative changes in the ovarian surface epithelium, suppression
of serum progesterone, atrophy of thymic cortex, a reduction in the number of lymphocy
tes, degenerative changes in the ovaries and kidney and degenerative changes in the liv
er compatible with porphyria tarda. IARC has concluded that while there is inadequate e
vidence for the carcinogenicity of HCB in humans, there is sufficient evidence in exper
imental animals. IARC has classified HCB as a possible human carcinogen (Group 2B).
HCB is unlikely to cause direct toxicological effects in aquatic animals at or below
saturation concentrations (approximately 5 µg/L) in water. At an exposure concentrati
on of 4.8 µg HCB/L for 32 days, there was no observed effect on embryonic through juv
enile stages in developing fathead minnows (Pimephales promelas) giving a NOEC of 4.8
µg/L. The caldoceran Daphnia magna, the amphipods Hylella azeteca, and Gammarus lacu
stris, the annelid worm Lumbricus variegatus, and the fathead minnow Pimephales prome
las were exposed to HCB at saturation concentration (5 µg/L) for 68 days. No effects
on survival, growth or reproduction were observed. Adult Japanese quail (Coturnix jap
onica) were fed diets containing HCB for 90 days, resulting in increased mortality at
100 µg/g diet and significantly reduced hatchability at 20 µg/g. At 5 µg/g increased
liver weight, slight liver damage and increased faecal excretion of coproporphyrin w
ere observed. Experiments conducted in mink (Mustela vison) and European ferrets (Mus
tela putorius furo) with dietary HCB resulted in adult mortality are higher doses (12
5 and 625 mg HCB/kg diet) and decreased litter size, increased percentage of stillbir
ths, increased kit mortality and decreased kit growth. Mink were generally more susce
ptible than ferrets to the effects of HCB. Results from another study indicate that i
n utero exposure to HCB resulted in higher kit mortality than exposure via the mothers
milk. HCB is very persistent. Estimated half lives in soil from aerobic and anaerobic
degradation range from 2.7 to 22.9 years. This persistence, combined with a high part
ition coefficient (log KOW = 3.03-6.42), provides the necessary conditions for HCB to
bioconcentrate in organisms. Bioconcentration factors of 22,000 and 106,840 have been
reported in fathead minnows and Lumbricus variegatus respectively. The chemical proper
ties of HCB (low water solubility, high stability, and semi-volatility) favour its lon
g range transport, and HCB has been detected in arctic air, water and organisms. HCB
is ubiquitous in the environment, and has been measured in foods of all types. HCB was
one of two organochlorines detected in all samples of Spanish meat and meat products s
urveyed with mean levels ranging from 8 ppb (fat weight) in pork products (cured ham) t
o 49 ppb in lamb, with a maximum level of 178 ppb in lamb. HCB was detected in 13 of 24
1 serum samples from Colorado beef cattle in a monitoring program, with an average conc
entration of 3.1 ppb. A survey of US pasteurized milk detected HCB in 8 of 806 composit
e milk samples. A survey of foods from India found average concentrations of HCB rangin
g from 1.5 ng/g (fat weight) in both oils and milk to 9.1 ng/g in fish and prawns, with
a maximum concentration of 28 ng/g in fish and prawns and an estimated daily intake of
0.13 µg/person. Average HCB residues in foods from Vietnam ranged from 0.28 ng/g (fat
weight) in pulses to 27 ng/g in caviar, with an estimated daily intake of 0.10 µg/person.
6.8 HEPTACHLOR
Chemical properties:
CAS Chemical Name: 1,4,5,6,7,8,8-Heptachloro-3a,4,7,7a-tetrahydro-4,7-methanol-1H-
indene.
Synonyms and Trade Names (partial list): Aahepta, Agroceres, Baskalor, Drinox,
Drinox H-34, Heptachlorane, Heptagran, Heptagranox, Heptamak, Heptamul, Heptasol,
Heptox, Soleptax, Rhodiachlor, Veliscol 104, Veliscol heptachlor.
CAS No.: 76-44-8; molecular formula: C10H5Cl7; formula weight: 373.32.
Appearance: White to light tan, waxy solid or crystals with a camphor-like odour.
Properties: Melting point: 95-96 C (pure), 46-74 C (technical); boiling point: 135-145 C
at 1-1.5 mm Hg, decomposes at 760 mm Hg; KH; 2.3 x 10 -3 atmámm3/mol; log KOC: 4.38; log
KOW; 4.40-5.5; solubility in water: 180 ppb at 25 C; vapor pressure: 3 x 10-4 mm Hg at 20
C. Heptachlor is a non-systemic stomach and contact insecticide, used primarily against
soil insects and termites. It has also been used against cotton insects, grasshoppers, s
ome crop pests and to combat malaria. Heptachlor is highly insoluble in water, and is s
oluble in organic solvents. It is quite volatile and can be expected to partition into
the atmosphere as a result. It binds readily to aquatic sediments and bioconcentrates i
n the fat of living organisms. Heptachlor is metabolised in animals to heptachlor epoxi
de, whose toxicity is similar to that of heptachlor, and which may also be stored in an
imal fat. The use of heptachlor has been banned in Cyprus, Ecuador, the EU, Portugal, S
ingapore, Sweden, Switzerland and Turkey. Its use is severely restricted in Argentina,
Israel, Austria, Canada, Czechoslovakia, Denmark, Finland, Japan, New Zealand, Philippi
nes, USA and USSR. There is no information on accidental or suicidal intoxication by h
eptachlor in humans. Symptoms in animals include tremors and convulsions. A study of wo
rkers from a plant involved in the production of heptachlor and endrin found a signific
ant increase in bladder cancer . This result was unexpected as no know bladder carcinog
ens were used at the plant, however, the small number of deaths (3) makes interpretatio
n of these findings difficult. No deaths from liver or biliary tract cancer were observ
ed, although mortality from cerebrovascular disease was higher than expected. There is
limited evidence that cyclodienes such as heptachlor may affect immune responses. The
acute oral LD50 of heptachlor to laboratory animals is in the range of 40 mg/kg body we
ight in rats to 116 mg/kg in rabbits. Groups of male and female rats were administered
daily doses of heptachlor orally beginning at 4 months of age, and continuing for 200
days. All the animals in the 50 and 100 mg/kg groups died by the 10th day of exposure.
Three animals in the 5 mg/kg group and 1 in the control died before the end of the study.
Beginning on the 50th day to the study, hyper-reflexia, dyspnoea and convulsions were
observed in the rats exposed to 5 mg/kg. Histological examination revealed fatty degene
ration of the liver cells and moderate fatty infiltration of the epithelium of the renal
tubules in the 5 mg/kg exposed group. In a reproduction study, rats were fed diets con
taining heptachlor in their diet throughout three generations. Mortality of pups in the
10 mg/kg group was slightly increased during the second and third weeks after birth in t
he second generation only. No adverse effects were observed in the lower dose levels. WH
O has reported no evidence of teratogenicity of heptachlor in rats and rabbits. IARC has
concluded that, while there is inadequate evidence for the carcinogenicity of heptachlo
r in humans, there is sufficient evidence in experimental animals. IARC has classified h
eptachlor as a possible human carcinogen (Group 2B). Heptachlor has been strongly impli
cated in the decline of several wild bird populations including Canada geese and the Ame
rican Kestrel in the Columbia Basin in the US. A population of Canada geese at the Umati
lla National Wildlife Refuge in Oregon experienced lowered reproductive success, and adu
lt mortality. Heptachlor epoxide residues were detected in the brains of dead birds and
in the eggs of nests with low success. The reproductive success of American Kestrels in
the same area was also reduced. Heptachlor epoxide residues in the eggs were associated
with reduced productivity. The presence of residues in the eggs indicates that heptachlo
r is transferred through the food chain, as Kestrels are not seed eaters, which was the
presumed route of exposure for the geese. Concentrations on the treated seeds were lower
than the recommended usage level indicating that effects on wildlife may occur, even if
heptachlor is used responsibly. Mink were fed diets containing heptachlor for 28 days,
followed by a 7 day recovery period to determine the subacute toxicity of heptachlor to
mink. The NOEL for mortality was 50 mg/kg (5.67 mg/kg body weight/day). Signs of toxici
ty including reduced food consumption and loss of body weight were observed in mink fed
the 25 mg/kg diet. In another study, adult male and female mink were fed diets containin
g heptachlor for 181 days (before and during the reproductive period) to determine effec
ts on reproduction. All the mink fed diets containing 25 µg/g (male and female) died, wi
thin 88 and 55 days respectively. The LOAEL, based on reduced kit growth, was 6.25 µg/g.
The half life of heptachlor in temperate soil is up to 2 years. This persistence, comb
ined with a high partition coefficient (KOW = 4.4-5.5), provides the necessary condition
s for heptachlor to bioconcentrate in organisms. Bioconcentration factors of heptachlor
and heptachlor epoxide in fathead minnows (Pimephales promelas) were 9,500 and 14,400, r
espectively. The chemical properties of heptachlor (low water solubility, high stability
, and semi-volatility) favour its long range transport, and heptachlor and its epoxide h
ave been detected in arctic air, water and organisms. WHO suggests that food is the maj
or source of exposure of heptachlor to the general population. Heptachlor has been detec
ted in the blood of cattle from both the US and Australia. Heptachlor was detected in 30
of 241 samples in American cattle, and violations of the MRL for heptachlor were detect
ed in 0.02 % of Australian cattle. In both instances, heptachlor was among the most freq
uently detected organochlorine. A daily intake of 0.25 µg/person/day (for heptachlor and
heptachlor epoxide combined, based on a 60 kg person) was estimated for Vietnam, and of
0.07 µg/person/day (for heptachlor alone) for India.
6.9 MIREX
Chemical properties:
CAS chemical name: 1,1a,2,2,3,3a,4,5,5a,5b,6-dodecachloroacta-hydro-1,3,4-metheno-1H-
cyclobuta[cd]pentalene
Synonyms and Trade Names (partial list): Dechlorane, Ferriamicide, GC 1283
CAS No.: 2385-85-5; molecular formula: C10Cl12; formula weight: 545.5
Appearance: White crystalline, odourless solid;
Properties: Melting point: 485 C; vapour pressure: 3 x 10-7 mm Hg at 25 C. Mirex is a
stomach insecticide with little contact activity. It's main use was against fire ants i
n the southeastern United States, but it has also been used to combat leaf cutters in S
outh America, harvester termites in South Africa, Western harvester ants in the US, mea
lybug of pineapple in Hawaii and has been investigated for possible use against yellow
jacket wasps in the US. It has also been used as a fire retardant in plastics, rubber,
paint paper and electrical goods. Mirex is very resistant to breakdown, is very insolub
le in water and has been shown to bioaccumulate and biomagnify. Due to its insolubility
, mirex binds strongly to aquatic sediments. There are no reports of injuries to human
s resulting from exposure to mirex. Mirex residues in human adipose have been reported.
A range of 0.16 - 5.94 ppm was reported in 6 of 1,400 samples collected in 1971-1972 i
n the southern US. Samples from 8 southeastern US states were collected, and residues d
etected in 10.2 percent of those tested, with a geometric mean of 0.286 ppm in lipid.
In acute studies, the oral LD50 of mirex to rats ranges from 600 to >3,000 mg/kg, depen
ding on sex of the test animal and nature of the formulation tested. Short term effects
included decreased body weight, hepatomegaly, induction of mixed function oxidases, an
d morphological changes in liver cells. Rats which were fed 5 ppm mirex in their diets
for 30 days prior to mating and for 90 days after, showed reduced litter size and incre
ased parental mortality. Reduced litter sizes, and viability of neonates, along with fo
rmation of cataracts were observed in rats fed 25 ppm mirex in the diet. IARC has concl
uded that while there is inadequate evidence for the carcinogenicity of mirex in humans
, there is sufficient evidence in experimental animals. IARC has classified mirex as a
possible human carcinogen (Group 2B). A reduction in germination and emergence in seve
ral plant species was observed, which increased as the concentrations of mirex increase
d. Uptake, accumulation and translocation of mirex by a variety of plant species has al
so been seen. These results are questionable, however, as lipophilic compounds such as
mirex are generally not known to be taken up and translocated by plants. Contamination
of plants is primarily a surface phenomenon resulting from aerial deposition of emissio
ns or deposition of compound that has volatilized from the surface of the soil. Crusta
ceans are the most sensitive aquatic organisms, with larval and juvenile stages being t
he most sensitive. Delayed mortality is typical of mirex poisoning in crustaceans. Larv
al crabs exposed to 0.1 and 10 µg/L did not exhibit any adverse effects on survival for
5 days after hatching. Delayed mortality then occurred at the 1 and 10 µg/L exposure l
evels. Mirex is also toxic to fish and can affect fish behaviour. Mirex has a low short
term toxicity to birds with acute oral LD50 values in the range of 1,400 mg/kg body we
ight in pheasant to 10,000 mg/kg in quail. Mirex is considered to be one of the most s
table and persistent pesticides, with a half life of up to 10 years. This persistence,
combined with lipophilicity, provides the conditions necessary for mirex to bioconcentr
ate in organisms. Bioconcentration factors of 2,600 and 51,400 have been observed in pi
nk shrimp and fathead minnows, respectively. The chemical properties of mirex (low wate
r solubility, high lipid solubility, high stability, and semi-volatility) favour its lo
ng range transport, and mirex has been detected in arctic freshwater and terrestrial or
ganisms. The main route of exposure of mirex to the general population is through food,
especially meat, fish and wild game, and intake is generally below established residue
tolerances. Mirex residues were found in only one of 806 milk sample composites collect
ed in a survey of US pasteurized milk. No residues of mirex were detected in any samples
of fish in Egypt nor in any samples from the fat of domestic farm animals in Ontario,
Canada.
6.10 POLYCHLORINATED BIPHENYLS
Chemical properties:
Trade Names for different mixtures (partial list): Aroclor, Pyranol,
Pyroclor, Phenochlor, Pyralene, Clophen, Elaol, Kanechlor, Santotherm, Fenchlor,
Apirolio, Sovol.
CAS No.: 1336-36-3 Congener Group Molecular Vapour Water
log KOW weight Pressure Solubility
(g/molecular) (Pa) (g/m3) Monochlorobiphenyl 188.7
0.9-2.5 1.21-5.5 4.3-4.6 Dichlorobiphenyl 223.1
0.008-0.60 0.06-2.0 4.9-5.3 Trichlorobiphenyl 257.5 0.003
-0.22 0.015-0.4 5.5-5.9 Tetrachlorobiphenyl 292.0 0.002
0.0043-0.010 5.6-6.5 Pentachlorobiphenyl 326.4 0.0023-0.051
0.004-0.02 6.2-6.5 Hexacholorbiphenyl 360.9 0.0007-0.012 0.0004
-0.0007 6.7-7.3 Heptachlorobiphenyl 395.3 0.00025 0.000045-0.
000 6.7-7 Octachlorobiphenyl 429.8 0.0006 0.0002-0.0003
7.1 Nonachlorobiphenyl 464.2 - 0.00018-0.0012 7.2-8
.16 Decachlorobiphenyl 498.7 0.00003 0.000001-0.000 8.26
Polychlorinated biphenyls (PCBs) are mixtures of chlorinated hydrocarbons that have been
used extensively since 1930 in a variety of industrial uses, including as dielectrics in
transformers and large capacitors, as heat exchange fluids, as paint additives, in carbo
nless copy paper and in plastics. The value of PCBs for industrial applications is relate
d to their chemical inertness, resistance to heat, non-flammability, low vapour pressure
and high dielectric constant. There are 209 possible PCBs, from three monochlorinated iso
mers to the fully chlorinated decachlorobiphenyl isomer. Generally, the water solubility
and vapour pressure decrease as the degree of substitution increases, and the lipid solub
ility increases with increasing chlorine substitution. PCBs in the environment may be exp
ected to associate with the organic components of soils, sediments, and biological tissue
s, or with dissolved organic carbon in aquatic systems, rather than being in solution in
water. PCBs volatilize from water surfaces in spite of their low vapour pressure, and par
tly as a result of their hydrophobicity; atmospheric transport may therefore be a signifi
cant pathway for the distribution of PCBs in the environment. The toxicology of PCBs is
affected by the number and position of the chlorine atoms, as substitution in the ortho p
osition hinders the rotation of the rings. PCBs without ortho substitution are generally
referred to as coplanar and all others as noncoplanar. Coplanar PCBs, like dioxins and fu
rans, bind to the AL-receptor and may exert, thus, dioxin-like effects, in addition to AL
-receptor independent effects which they share with non-coplanar PCBs (e.g. tumor promoter)
. Association between elevated exposure to PCB mixtures and alterations in liver enzymes,
hepatomegaly, and dermatological effects such as rashes and acne has been reported. Ad
verse effects are predominantly associated with higher blood concentrations. Contamina
tion of rice oil by PCBs in Japan (1968) and Taiwan (1979) has resulted in the exposure
of a large number of people to PCBs and their contaminants PCDFs. Signs and symptoms o
f exposure from these incidents include enlargement and hyper secretion of the Meibomia
n glands of the eyes, swelling of the eyelids, and pigmentation of the nails and mucous
membranes, occasionally associated with fatigue, nausea and vomiting. This was followe
d by hyperkeratosis and darkening of the skin with follicular enlargement and acneform
eruptions, often with a secondary staphylococcal infection. Children born up to 7 years
after maternal exposure in the Taiwan incident had hyperpigmentation, deformed nails a
nd natal teeth, intrauterine growth delay, poorer cognitive development up to 7 years o
f age, behavioural problems and higher activity levels. The affected children appeared
to "catch up" to controls at 12 years of age. Children born seven to twelve years after
maternal exposure experienced mildly delayed development, but no differences in behavi
our. Effects observed in these children is likely a result of the persistence of PCBs i
n the human body, resulting in prenatal exposure long after the exposure took place. Th
ese effects are consistent with the observations of poorer short term memory functionin
g in early childhood, in children exposed prenatally by mothers who had high consumptio
n of Lake Michigan sports fish containing PCBs, amongst other POPs. People exposed in
the Yucheng incident had low resistance, and suffered from a variety of infections. Exa
mination during the first year revealed decreased concentrations of IgM and IgA, decrea
sed percentages of total T-cells, active T-cells and helper T-cells, but normal percent
ages of B-cells and suppressor T-cells; suppression of delayed type response to recalli
ng antigens; enhancement of lymphocyte spontaneous proliferation and an enhancement in
lymphoproliferation to certain mitogens. After three years, some, although not all, of
the effects had disappeared. Cancer deaths in both male and female workers involved in
the manufacture of electrical capacitors were significantly increased. A significant in
crease in haematological neoplasms and gastrointestinal cancers was observed in male wo
rkers. A non-significant increase in lung cancer was observed. The study was, however,
limited by the small numbers of deaths. PCBs have a low acute toxicity to laboratory a
nimals, with acute oral LD50 values in rats in the range of 2 to 10 g/kg body weight. E
ffects are manifested primarily through chronic exposure. Effects on the liver, skin, i
mmune system, reproductive system, gastrointestinal tract and thyroid gland have been o
bserved associated with exposure to PCB mixtures or individual congeners. Adverse repro
ductive effects observed in several studies on monkeys exposed to PCBs include low birt
h weights, skin hyperpigmentation, behavioural disturbances, atrophy of the thymus and
lymph nodes, bone marrow hypoplasia and hyperplasia of the gastric mucosa. Female rhesu
s monkeys fed diets containing Aroclor 1016 in the diet were bred after 7 months of die
tary exposure. Neonatal weights in the 1.0 ppm group were significantly decreased. PCBs
have not been observed to be teratogenic in studies involving rats and non-human prima
tes when tested orally, during critical periods of organogenesis. A moderate but statis
tically significant inhibitory effect on the immune system of rhesus monkeys has been o
bserved, resulting from chronic, low level exposure to Aroclor 1254 and that these effe
cts may be due to altered T-cell and/or macrophage function. IARC has concluded that th
ere is limited evidence for the carcinogenicity of PCBs in humans, and there is suffici
ent evidence in experimental animals. PCBs are therefore classified as probable humans
carcinogens (Group 2A). PCBs are toxic to aquatic organisms, with 96-hour LC50 values
in the range of 0.015 mg/L in fathead minnows to 2.74 mg/L in bluegills. Fathead minnow
s were exposed to Aroclor 1242, 1248 or 1254 in a continuous flow bioassay for 9 months
. Reproduction occurred at and below 5.4 µg Aroclor 1242/L, however, results were highly
variable. A significant reduction in spawning was observed in fish exposed to 1.8 µg
Aroclor 1254/L. Early life stages of fish are more sensitive to the effects of dioxins,
furans, and PCBs. Parts per trillion concentrations of these structurally related chem
icals in lake trout and rainbow trout eggs produce toxicity through sac fry mortality a
ssociated with yolk sac edema and haemorrhages. PCBs have a low acute toxicity to bird
s, with 5-day dietary LC50 values in the range of 747 mg/kg diet in quail to >5,000 mg/
kg in several species. Broiler breeder and leghorn hens who were fed diets Aroclor 1242
for one week experienced reduced hatchability and the effects continued after exposure
was terminated. There is growing evidence linking persistent halogenated aromatic hyd
rocarbons such as PCBs to reproductive and immunotoxic effects in wildlife. Two groups
of 12 female seals (Phoca vitulina) were fed diets of fish from the western part of the
Wadden Sea, or from the north-east Atlantic. Residue analysis showed statistically sig
nificant differences between the two diets for PCBs and DDE. The average daily intake f
or group 1 was 1.5 mg PCBs and 0.4 mg DDE, and 0.22 mg and 0.13 mg for group 2. Females
were mated with undosed males and reproductive success was significantly lower in grou
p 1. Mink fed Lake Michigan Coho salmon containing between 10 and 15 ppm PCBs as 30% of
their diet for five months failed to whelp as did those fed a diet containing 5 ppm Ar
oclor 1254. The clinical signs and lesions observed in mink fed a diet containing Lake
Michigan coho salmon included anorexia, bloody stools, fatty liver, kidney degeneration
and gastric ulcers, and were similar to those fed a diet supplemented with PCBs. The
degradation of PCBs in the environment depends largely on the degree of chlorination of
the biphenyl, with persistence increasing as the degree of chlorination increases. Hal
f-lives for PCBs undergoing photodegradation range from approximately 10 days for a mon
ochlorobiphenyl to 1.5 years for a heptachlorobiphenyl. The persistence of PCBs, combin
ed with the high partition coefficients of various isomers (log KOW ranging from 4.3 to
8.26) provide the necessary conditions for PCBs to bioaccumulate in organisms. Bioconc
entration factors of 120,000 and 270,000 have been reported in fathead minnows. Concent
ration factors in fish exposed to PCBs in their diet were lower than those for fish exp
osed to PCBs in water, suggesting that PCBs are bioconcentrated (taken up directly from
the water) as opposed to being bioaccumulated (taken up by water and in food). The che
mical properties of PCBs (low water solubility, high stability, and semi-volatility) fa
vour their long range transport, and PCBs have been detected in arctic air, water and o
rganisms. The main source of PCB exposure to the general population is through food, e
specially fish. PCB residues were detected in 8.5% of samples, with a maximum of 0.30 m
g/kg fat, taken during a survey of the fat of domestic farm animals in Ontario, Canada
between 1986 and 1988. In a survey of foods in Vietnam, the highest levels of PCBs were
detected in fish and shellfish, with levels of 760 and 1,400 ng/g fat. The main source
s of PCBs in the Vietnamese diets is cereals (including rice) and vegetables, and the d
aily intake of 3.7 µg/person/day is comparable to those of some industrialized countrie
s . A survey of foods in India also found that the highest levels of PCBs were in fish,
with an average of 330 ng/g fat. Again, the main source of PCB dietary intake (0.86 µg
/person/day) was cereal and vegetable oil.
6.11 TOXAPHENE
Chemical properties:
CAS Chemical Name: Toxaphene
Synonyms and Trade Names (parital list): Alltex, Alltox, Attac 4-2, Attac 4-4, Attac 6,
Attac 6-3, Attac 8, Camphechlor, Camphochlor, Camphoclor, Chemphene M5055, chlorinated
camphene, Chloro-camphene, Clor chem T-590, Compound 3956, Huilex, Kamfochlor, Melipax,
Motox, Octachlorocamphene, Penphene, Phenacide, Phenatox, Phenphane, Polychlorocamphene,
Strobane-T, Strobane T-90, Texadust, Toxakil, Toxon 63, Toxyphen, Vertac 90%.
CAS No.: 8001-35-2; molecular formula: C10H10Cl8; formula weight: 413.82
Appearance: Yellow, waxy solid with a chlorine/terpene-like odour.
Properties: Melting point: 65-90 C; boiling point: >120 C (decomposes); KH: 6.3 x 10-2
atmám3/molecular; log KOC: 3.18 (calculated); log KOW: 3.23-5.50; solubility in water:
550 µg/L at 20 C; vapour pressure: 0.2-0.4 mm Hg at 25 C. Toxaphene is a nonsystemic a
nd contact insecticide that was used primarily on cotton, cereal grains fruits, nuts an
d vegetables. It has also been used to control ticks and mites in livestock. Toxaphene
has been in use since 1949 and was the most widely used insecticide in the USA in 1975.
Toxaphene is highly insoluble in water, and has a half life in soil of up to 12 years
It has been shown to bioconcentrate in aquatic organisms and is known to undergo atmos
pheric transport. Toxaphene has been banned in 37 countries, including Austria, Belize,
Brazil, Costa Rica, Dominican Republic, Egypt, the EU, India, Ireland, Kenya, Korea, M
exico, Panama, Singapore, Thailand and Tonga. Its use has been severely restricted in 1
1 other countries, including Argentina, Columbia, Dominica, Honduras, Nicaragua, Pakist
an, South Africa, Turkey and Venezuela. In a human volunteer study, twenty-five subjec
ts were exposed to approximately 1 mg toxaphene/kg body weight/day in a closed chamber
to an aerosol of toxaphene for a total of 13 days. Physical examination, blood and urin
e tests did not reveal any toxic effects. In a separate study, eight women working in a
n area that had been sprayed with toxaphene at a rate of 2 kg/ha had a higher incidence
of chromosome aberrations (acentric fragments and chromatid exchanges) than in control
individuals. Annual physical examination of 137 workers involved in the manufacture of
toxaphene did not reveal adverse effects associated with the exposure . Similarly, a m
ortality survey of 199 employees who had worked with toxaphene found that none of the d
eaths appeared to be directly related to the exposure. The acute oral toxicity of toxa
phene is in the range of 49 mg/kg body weight in dogs to 365 mg/kg in guinea pigs. In a
13 week study, rats were fed diets containing toxaphene. Liver/body weight ratio and h
epatic microsomal enzyme activities were increased in rats fed 500 ppm. Dose dependent
histological changes were observed in the kidney, thyroid and liver. The NOAEL was dete
rmined to be 4.0 ppm (0.35 mg/kg). In another study, beagle dogs were fed toxaphene for
13 weeks. The liver/body weight ratio and serum alkaline phosphatase were increased in
dogs fed 5.0 mg/kg. Mild to moderate dose dependent histological changes were observed
in the liver and thyroid. The NOAEL for dogs was determined to be 0.2 mg/kg. Male and
female rats were fed toxaphene in their diets for a total of 13 weeks in a one generati
on, two litter reproduction study. Effects in both the F0 and F1 adults at levels from
20 to 500 ppm included increased liver and kidney weight, and histological changes in t
he thyroid, liver and kidney. IARC has concluded that while there is inadequate evidenc
e for the carcinogenicity of toxaphene in humans, there is sufficient evidence in exper
imental animals. IARC has classified toxaphene as a possible human carcinogen (Group 2B
). Toxaphene is essentially nontoxic to plants. In general, toxic effects have been ob
served only at levels much higher than the recommended usage level. Toxaphene is highly
toxic, with 96-hour LC50 values in the range of 1.8 µg/L in rainbow trout to 22 µg/L i
n bluegill. Brook trout exposed to toxaphene for 90 days experienced a 46% reduction in
weight at 0.039 µg/L, the lowest concentration tested. Egg viability in female trout w
as significantly reduced upon exposure to a concentration of 0.075 µg/L or more. Long t
erm exposure to 0.5 µg/L reduced egg viability to zero. Female ring-necked pheasants ex
posed to 300 mg toxaphene/kg diet experienced reductions in egg laying and hatchability
. The half-life of toxaphene in soil ranges from 100 days up to 12 years, depending on
the soil type and climate. This persistence, combined with a high partition coefficien
t (log KOW = 3.23-5.50) suggests that toxaphene is likely to bioconcentrate. Bioconcent
ration factors of 4,247 and 76,000 have been recorded in mosquito fish and brook trout,
respectively. The chemical properties of toxaphene (low water solubility, high stabili
ty, and semi-volatility) favour its long range transport, and toxaphene has been detect
ed in arctic air. Exposure of the general population is most likely through food, howev
er levels detected are generally below maximum residue limits. Due to its being banned
in many countries, recent food surveys have generally not included toxaphene and hence
recent monitoring data are not available.
Back
For more detail:
The source of this data is IPCS
[The International Programme on Chemical Safety, within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals (IOMC)], by
L. Ritter, K.R. Solomon and J. Forget of the Canadian Network of Toxicology Centres, and by M. Stemeroff and C.O'Leary of the Deloitte and Touche Consulting Group.
|

